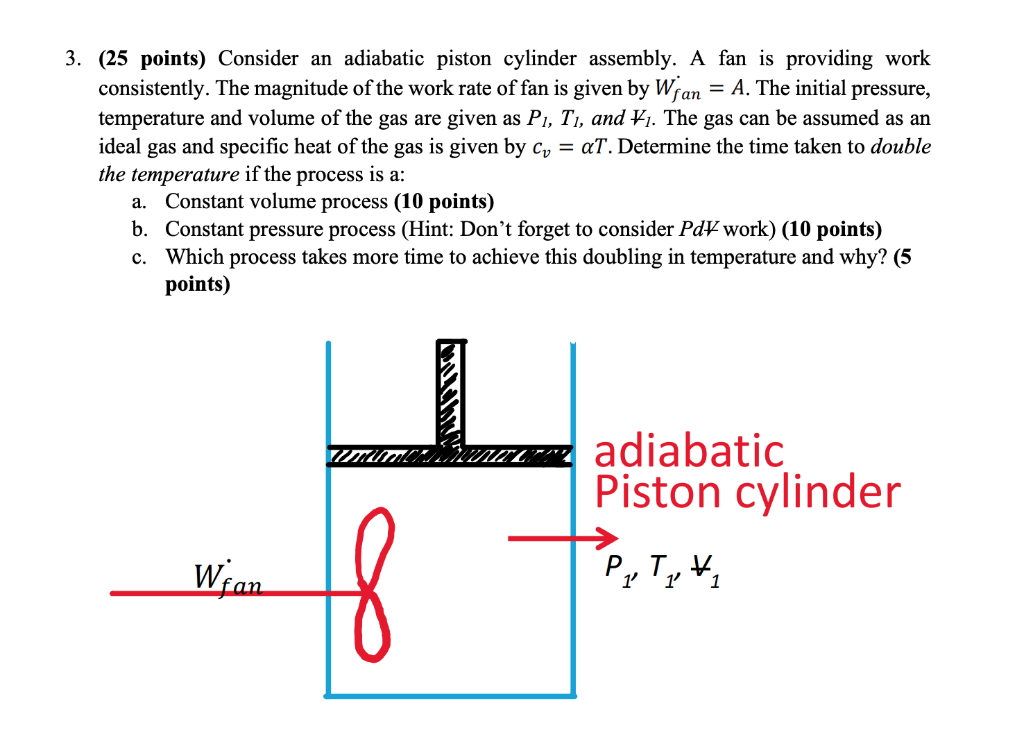
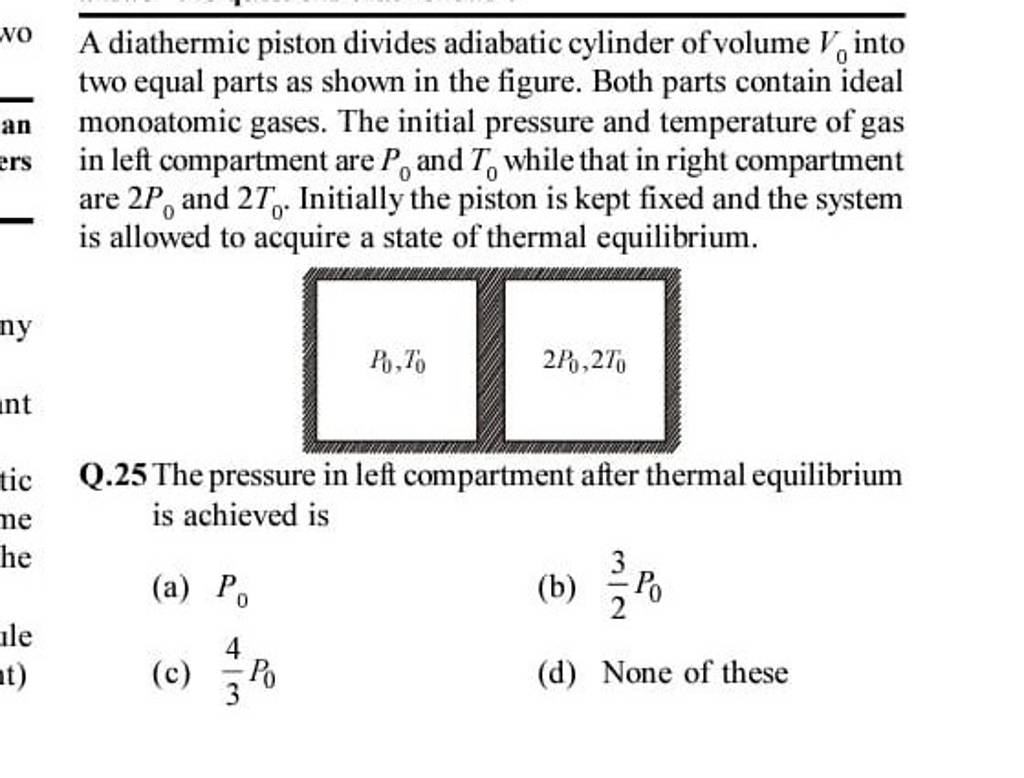
An adiabatic cylinder of volume V0 is divided by an adiabatic piston in two equal compartments. Both the compartments are filled with ideal monoatomic gas at pressure and temperature P0, T0 and

A)-(D) address the adiabatic compression of an ideal gas in a piston (see figure). The system is the gas inside the piston. You can assume a negligible change in the center of

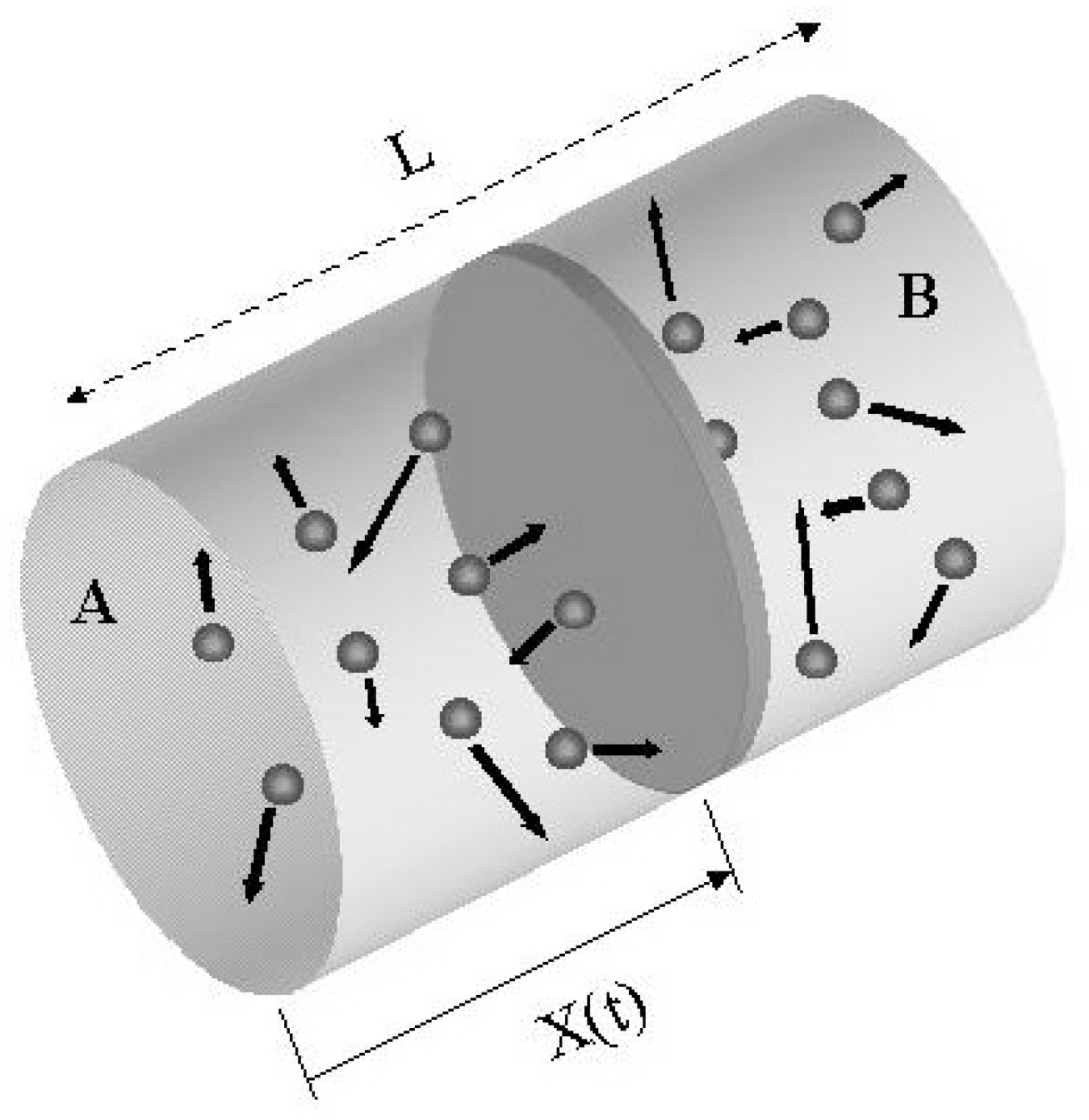
A cylindrical container of volume 44.8 L is containing equal number of moles of an ideal monoatomic gas in two sections A and B, separated by an adiabatic frictionless piston as shown

Ideal Adiabatic Process of A Piston Expanding (Find Work and Total Internal Energy Change) - YouTube

Air is compressed in an adiabatic piston-cylinder device from 295 K and 95 kPa in an isentropic manner. If the compression ratio, V1/V2 of this piston-cylinder device is 8. Assume air behaves

An adiabatic piston of mass m equally divides an insulator container of length l. One end of alight spring is connected to the piston and other end to the right wall. The